Shimadzu has produced a technical note describing an innovative solution for ensuring data integrity for spectroscopy systems.
A recent topic related to analytical data is the lack of data integrity due to data modification and replacement. Regulatory authorities for analytical instruments are not only interested in chromatography systems, such as liquid chromatographs (LC) and gas chromatographs (GC), but are also turning their interest to spectroscopy systems, such as UV and IR systems. Consequently, many analytical laboratories are urgently considering how to ensure data integrity for spectroscopy systems. This report describes an innovative solution for ensuring data integrity for such spectroscopy systems.
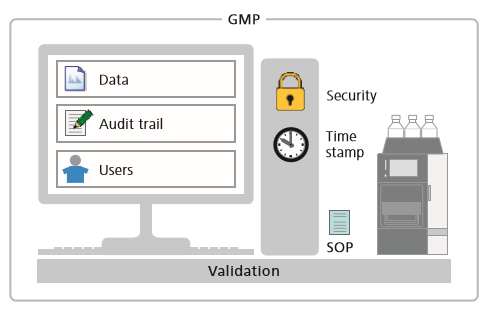 Topics addressed in this technical note include:-
Topics addressed in this technical note include:-
- References to Spectroscopy Systems by Regulatory Authorities
- FDA Warning Letters
- Data Integrity Compliance for Spectroscopy Systems
- Obstacles for Ensuring Data Integrity Compliance for Spectroscopy Systems: Audit Trail and User Management
- Innovative Data Integrity Compliance
- Procedure for Using LabSolutions to Create a Report Set for Spectroscopy Systems
View the on-demand webinar on 'Assuring Laboratory Data Integrity in a Time of Enhanced Regulatory Oversight' by David Stokes (Principal Consultant and Director, Convalido Consulting Ltd, Newcastle-Upon-Tyne, UK) and be able to download the technical note.




