The separation power of HPLC combined with the very high sensitivity and selectivity of MS detection is widely used to develop analytical methods that are required to achieve ultra-low detection and quantification limits. Examples of such demanding HPLC-MS applications comprise clinical and preclinical pharmacokinetic studies, proteomic studies and analytical methods for food safety and environmental monitoring.
HPLC is mainly hyphenated to MS detection with ESI (electrospray ionization) sources, capable of converting the compounds in solution coming from the HPLC in charged ions in the gas phase, which can enter into the high vacuum zone of the MS for the detection. In this process the quality of the formation of the gas phase ions is crucial for increasing the detection sensitivity. Without going into details, the gas phase ion generation can be described as a two-step process. The first one is the dispersion of the liquid into a fine aerosol of charged droplets assisted by high voltage conditions. The second one is the desolvation step, during which the solvent is evaporated and the size of charged droplet progressively decreases until leading to a stream of isolated gas phase ions, that can enter in the MS analyser.
HILIC chromatography, based on the use of mobile phase at high percentage of organic solvent, such as acetonitrile, offers unique advantages for mass spectrometric detection in comparison to reversed-phase chromatography, reflected in three main points:
- Increased ESI spray stability : Acetonitrile has a surface tension lower than water, requiring lower voltages for the generation of stable sprays. The use of lower voltages preserves the metal capillary form consumption and change in the diameter, which leads to poor spray stability. In addition, lower voltages avoid the possibility of electrical arcing in the source which may impair the instrument electronics. Stable sprays are one of the main requisites for the reproducibility of the analysis.
- More efficient solvent evaporation : The higher organic content of the eluent in HILIC supports a quicker and more complete solvent evaporation, which allows an efficient release of the gas phase ions. This improves the signal strength, thus enhancing the sensitivity.
- Alteration of ion suppression : Ion suppression is a phenomenon due to the matrix effect, where compounds of different nature (they can be solvent additives, matrix, or co-eluting analytes) co-elute with the analyte of interest, and compete in the electrospray droplet for the charges on the droplet surface. More polar and ionisable compounds are generally more prone to acquire the charge. When the analyte of interest is in the presence of such interfering compounds, the formation of its ion is suppressed, with consequent decrease of signal intensity. Polar compounds in RPLC are more prone to ion suppression because they elute in the first region of the chromatogram, where matrix is mainly present. HILIC shows a completely different selectivity in comparison to RPLC, so that polar compounds are better separated from the ion suppression region and can be more reliably quantified.
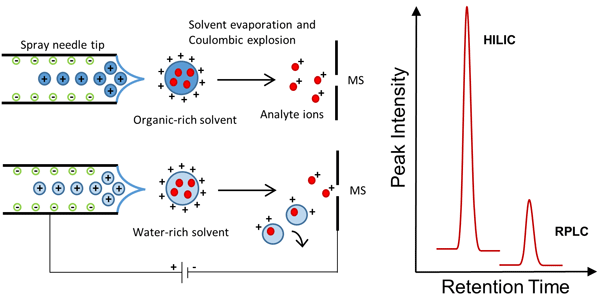
Figure 1: Ion generation in ESI under organic-rich HILIC conditions and under water-rich RPLC conditions. HILIC conditions allow a more efficient desolvation, thus a higher number of gas phase ions enter in the MS with ensuing increase of the sensitivity.
Finally, it has to be considered that the compounds analysed by HILIC are polar or ionic in nature, and this can assist the ion generation in the ESI process. Overall, it has been observed that the increase in sensitivity achieved with HILIC is on average around 10-fold.
From an experimental point of view, the buffer concentration recommended for HILIC-MS is around 10 mM. This concentration allows good salt solubility at high organic solvent, and it is a good compromise for efficient ion generation without extensive contamination of the ion source. It is worth remembering that just volatile buffers, such as ammonium formate, acetate or bicarbonate, are recommended for HILIC-MS applications.
In the forthcoming instalment, we will discuss different possibilities to combine RPLC and HILIC in order to get the benefits from both techniques.
This blog article series is produced in collaboration with Dr Giorgia Greco, Product Manager with Thermo Fisher Scientific in Germany and Thomas Letzel, Associate Professor and Head of the Analytical Research Group at the Technische Universität München, Germany.
Giorgia Greco received a PhD in Chemistry and worked as a Post Doc researcher at the Technische Universität München, Germany. During her research, she specialized in the fundamental of LC-MS and in the separation and analysis of metabolites from human and food matrices, as well as organic contaminants in waste water samples, by hyphenated HPLC/MS and HILIC/MS techniques.
Thomas Letzel received his PhD in Chemistry with Aerosol Analysis and then worked as a Post-Doc performing pharmaceutical analysis. He is the author of more than 50 publications and two books and wants to share his experience in liquid chromatography, especially in HILIC, with the community to accelerate the dissemination about HILIC theory and practical handling.
If you have any questions about this article send them to techtips@sepscience.com



