A reader observed baseline drift in his gradient method and wondered where it came from and what could be done to eliminate it. There are two main sources of baseline drift – temperature and mobile phase. If the column is operated in a column oven, the temperature should be constant, so temperature-related drift usually is not a problem, since most HPLC systems are configured with ovens.
Baseline drift related to the mobile phase can have two major factors influencing the magnitude of the drift – the solvent characteristics and the detection wavelength. Let’s look at each of these factors. With most reversed-phase methods, the weak solvent (A, water or buffer) usually has lower UV absorbance than the strong solvent (B; methanol, MeOH; acetonitrile, ACN; or tetrahydrofuran, THF). This effect can be seen in Figure 1 for two different solvents. In both cases, the A-solvent is 10 mM potassium phosphate (pH 2.8) and a 5-80% B gradient is run over 10 min. Notice the upward drift of the baseline at 215 nm in both examples. In Figure 1 with MeOH as the B-solvent, the drift is small (about 0.01 AU over the gradient), because MeOH absorbs UV light at 215 nm more strongly than does phosphate buffer. The magnitude of this drift is not large, because 10 mM phosphate has nearly the same absorbance as MeOH. If the same gradient were shown with water as the A-solvent, the drift would be much more pronounced (not shown). This is one trick to reduce baseline drift – just add a UV-absorbing compound to the A-solvent at the appropriate concentration so that its UV absorbance matches the B-solvent.
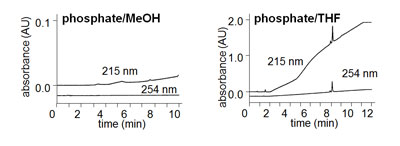
Figure 1
Contast the drift for MeOH with THF in Figure 1. THF has much stronger UV absorbance than MeOH at 215 nm, so the resulting baseline drift (≈2 AU vs. ≈0.01 AU) is much larger, too. It is unlikely that you could fully correct for this much drift by the addition of a UV-absorber to the A-solvent. This is just one reason why THF is not commonly used today. Its high UV absorbance at low wavelengths precludes its use for many applications.
Another major influence on baseline drift is the wavelength selected for UV detection. For both organic solvents, you can see that the drift is reduced or eliminated by moving from 215 nm to 254 nm. This is because MeOH and THF (and most organic solvents used in HPLC) have less UV absorbance at 254 nm than at 215 nm.
So there are two ways to reduce baseline drift in gradient methods. One way is to either increase the absorbance of the A-solvent by adding a UV-absorber or we can reduce the absorbance of the B-solvent by selecting a more transparent solvent. For example, ACN has lower absorbance at low wavelengths than either MeOH or THF, so it is favored as the B-solvent for methods that need to run at220 nm.
A final consideration is whether or not we need to do anything about baseline drift. If the drift is sufficiently small that all the peaks stay within the linear range of the detector (usually 0 – 1 AU, but sometimes more), there may not be any need to compensate for drift. Today’s data systems do quite well at integrating peaks appearing on drifting baselines. If the drift is excessive, such as with THF at 215 nm, we will probably have to do something to reduce the drift.
This blog article series is produced in collaboration with John Dolan, best known as one of the world’s foremost HPLC troubleshooting authorities. He is also known for his research with Lloyd Snyder, which resulted in more than 100 technical publications and three books. If you have any questions about this article send them to TechTips@sepscience.com




