Shifting retention times within a sample batch can be a real source of headaches in the routine HPLC laboratory. Previously we talked about the kind of retention drift experienced by some HPLC methods for the first few injections. This often is associated with slow equilibration of a mobile phase or sample component with the column, but usually disappears after the first three or four injections. We saw that often a large-concentration loading injection would speed this process and that ignoring the first injection or two was the simplest way to deal with this problem.
A reader (D.M.) reminded me that retention drift is most common with proteins and peptides, and that the most common symptom for small molecules is not retention drift, but peak-area drift. This happens as the active sites on the column bind some of the sample with each subsequent injection until the slow-equilibration sites are effectively saturated with sample. Then the peak areas are constant. Another form of retention drift is more insidious. I have seen this with methods that monitor pharmaceutical tablet dissolution. Recently a reader (T.S.) reported a similar problem in a completely unrelated system. He was analysing peptides on a nano-column and observed continuing shifts to longer retention times as additional samples were analysed. Figure 1 shows a portion of the chromatograms from two successive gradient runs. Retention times increased by approximately two minutes for each peak (arrow).
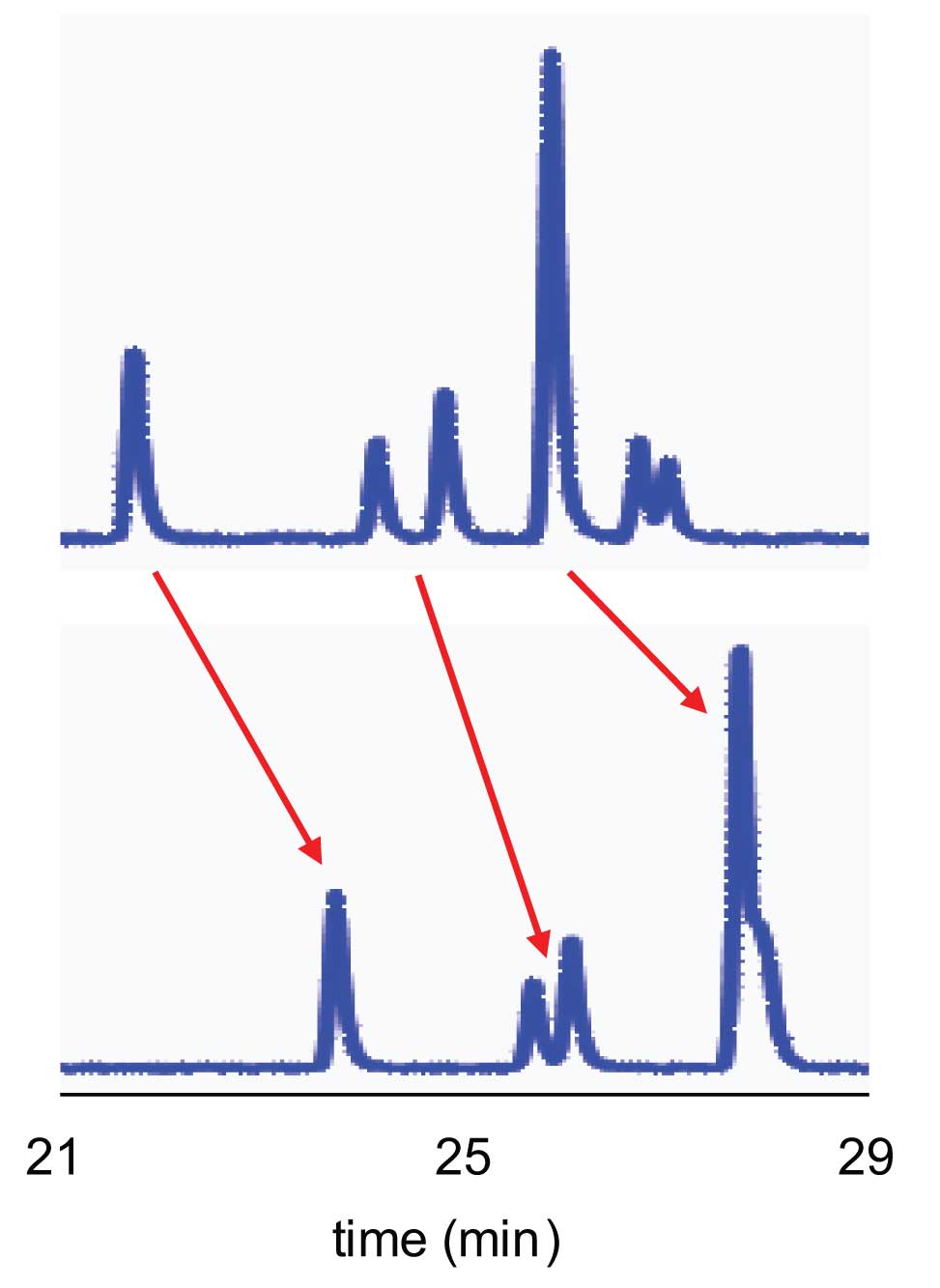 Figure 1
Figure 1
In the case of dissolution samples, I have received similar complaints – with each successive run, the retention times increased a bit. Dissolution baths are designed to simulate the dissolution of a drug tablet in the stomach, so the bath contains HCl, water, and a number of other additives to imitate the human stomach contents. One of the components of this magic potion is a surfactant, such as sodium lauryl sulfate (SLS).
Ion-pairing reagents often are added to a mobile phase to help increase the retention of ionic species. For example, a reagent containing both a hydrophobic group and a negative charge, such as hexane sulfonate, is added to retain cationic sample components. In one model of ion pairing, the hydrophobic end of the molecule is attracted to the hydrophobic surface of a reversed-phase column, leaving the ionized end of the molecule sticking out in the more polar mobile phase. This creates an in situ ion-exchange surface that is useful for retaining oppositely charged sample analytes.
One of the biggest problems with ion pairing is a result of the equilibrium between the ion-pairing reagent and the stationary phase. The equilibration process is very slow, and it is strongly influenced by the organic content of the mobile phase and the column temperature. Normally we think of the column equilibrating with the mobile phase in no more than about 10 column volumes. This means we can put a fresh mobile phase on the HPLC system and start the flow, and by the time we’re ready to make the first injection, the column generally is equilibrated. Not so with ion pairing – equilibration can take 20-50 column volumes or more to be complete. The sensitivity to mobile phase composition means that most ion-pairing applications are limited to isocratic mobile phases, where the organic content is constant. Gradient conditions might never be in equilibration.
Well, SLS is an ion-pairing reagent, too. And it is so non-polar, it sticks on reversed-phase columns quite strongly. Because dissolution-bath samples are quite clean, samples often are injected directly, perhaps with dilution or filtration as the only sample preparation steps. When this happens, each injection contains a little SLS, and the SLS gradually builds up on the column. Because the SLS is incrementally added to the column, the immobilized ion-pairing reagent concentration on the column increases with each subsequent injection. Equilibrium may be reached at some point, but it takes many, many injections, if it ever occurs. As a result, cationic samples, such as basic drugs, are retained not only by reversed-phase, as expected, but also by ion pairing. With a constantly changing load of ion-pairing reagent, retention may never stabilize.
In a follow-up email, T.S. indicated that he had tracked down the problem with peptide retention drift as being related to residual sodium dodecyl sulfate (SDS) that had carried through his sample cleanup process and remained in the injected sample solution. So in his case, the SLS was acting as an ion-pairing reagent, gradually building up on the column, and gradually increasing the retention of basic amino acids.
The solution to either of these problems? One would be to eliminate the surfactant from the process, but that wouldn’t be practical. A second technique might be to improve the sample preparation process to eliminate the surfactant – this might be a lot of work, but it is feasible. I think the thing I would try is to intentionally add the ion-pairing reagent to the mobile phase at a low concentration to see if complete equilibrium could be established, resulting in stable retention times. This last solution might be difficult to achieve with the peptide method that required gradient elution. It also might make unacceptable changes in selectivity. When unintentional ion pairing enters, the picture rarely is pretty!
This blog article series is produced in collaboration with John Dolan, best known as one of the world’s foremost HPLC troubleshooting authorities. He is also known for his ongoing research with Lloyd Snyder, resulting in more than 100 technical publications and three books. If you have any questions about this article send them to TechTips@sepscience.com




