We discussed the significance of a constant electro-osmotic flow and good capillary conditioning for precision in our CE methods. Now we look at the importance of injection settings for precision. We will also have a first look at sample stacking and see that by carefully selecting our sample solvent we can gain sensitivity in our applications.
The Two Principles of Injection
Sample injection in CE is basically done in two different ways: by pressure difference or by applying a voltage. Injecting by pressure difference is called hydrodynamic injection. The volume of sample injected depends not only on the injection time and pressure, but also on the BGE viscosity, the capillary diameter and length. The sample volume injected can be estimated by:
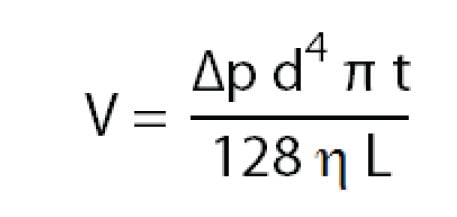
Injection by applying a voltage is called electrokinetic injection. In electrokinetic injection, the amount injected of a certain sample component depends on the component’s mobility, the capillary diameter, the applied electric field and the injection time. This means that sample components with different mobilities are injected in different amounts . Consequently, electrokinetic injection is a selective or discriminative injection mode and for electrokinetic injection, one cannot say that a certain volume of sample is being injected. The amount of a compound injected can be estimated by:
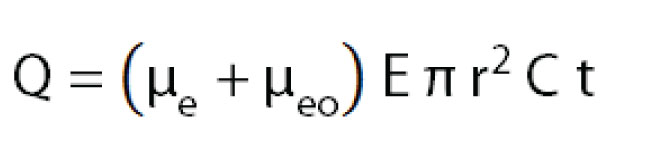
As sample injection for both injection modes depends on the capillary diameter and length, make sure to adjust the injection settings when testing different capillary dimensions.
Should I select hydrodynamic or electrokinetic injection?
Well, as usual, it depends. Generally speaking hydrodynamic injection is more precise than electrokinetic injection. However when working with viscous applications, hydrodynamic injection is cumbersome. An example is capillary gel electrophoresis (CGE). In CGE, the capillary is filled with a viscous gel buffer and usually electrokinetic injection is applied. Selectivity can be another reason for choosing electrokinetic injection. For instance if the analytes of interest are selectively injected into the capillary while the sample matrix components have a mobility in the opposite direction and remain in the sample vial. Selective injection also means that the amount injected of faster migrating compounds is bigger than the amount of slower migrating compounds. That is why quantitative analysis after electrokinetic injection requires appropriate calibration. Selective injection also implies that electrokinetic injection changes the composition of the remaining sample. So as a basic rule we can only inject once from a sample vial when applying electrokinetic injection.
Precision: The Details
For good precision injection, there are more details we need to pay attention to. As viscosities and therefore injected volumes and amounts can vary 2–3 % per degree Celsius, the samples and capillary should have a constant, precise temperature. Solutions that come from the fridge need some time to attain room temperature or the temperature of the sample carrousel. A way to make sure that all sample and BGE vials have had time to get at a constant temperature is to programme the capillary pre-sequence conditioning (1) as part of the sequence.
A small sample zone and short injection time reduce band broadening and maintain efficient peaks with high plate numbers. That is why the injection plug length should ideally be less than 1–2 % of the capillary length and why the injection time should be as short as possible. Simultaneously, the injection time is used in modern instruments to correct for pressure or voltage variability during injection. This improves injection volume precision but only works well if the injection time is at least 3 s.
Precise injection pressure differences and prevention of syphoning requires that the liquid levels in the vials are constant and the same. So fill all sample vials to the same levels. The inlet and outlet BGE vials have to be level. Do not put the waste vial at the outlet of the capillary when injecting, as this is probably the vial with the most variable liquid level.
To remove excess sample sticking on the outside of the capillary, dip the capillary after injection in a clean water or BGE vial (Figure 1). It is also advantageous to inject a BGE or water plug after sample injection to prevent sample loss by thermal expansion when the high voltage is switched on.
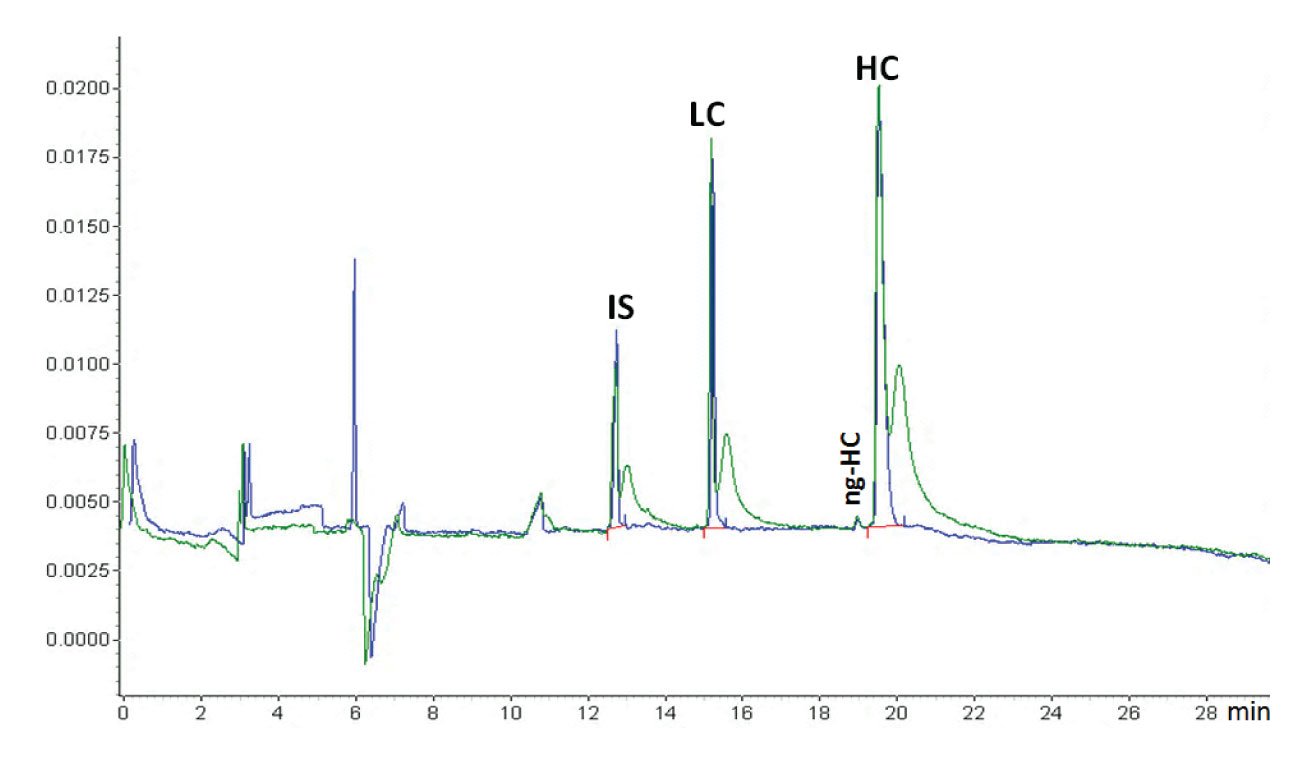 Figure 1: An example of the effect of dipping (blue trace) or not dipping (green trace) the capillary inlet in a clean water vial after sample injection (courtesy of Beckman Coulter). When the capillary was not dipped in water after sample injection, the sample was stuck at the outside of the capillary. This migrated into the capillary after the run was started and the voltage applied, causing the extra sample peaks just after the proper sample peaks. The sample is reduced IgG and the application is the Beckman Coulter CE-SDS kit. This is a CGE protein sizing application comparable with SDS-PAGE. The protein sample is treated with SDS for denaturisation and β-mercaptoethanol for reduction. The original IgG antibody molecule is thus reduced to its heavy chain (HC) and light chain (LC). Also, some non-glycosylated HC (ng-HC) is visible. Because of the denaturisation, all proteins are covered with equal amounts of SDS per amount of protein, shielding the natural charge of the protein. That means that all proteins have the same charge/size ratio. The capillary is filled with SDS-gel buffer which is a linear polymer that through entanglement creates a molecular size sieve and the separation is size-based, just as in SDS-PAGE. Because of the gel, sample injection is electrokinetic (although hydrodynamic injection is also feasible (5)). Because of the unified charge/size ratio, the electrokinetic injection is discriminative, but not between the different proteins. The function of the internal standard (IS), a 10 kDa protein, is for migration time correction. Quantification is by internal normalization (sum corrected peak areas equals 100 %).
Figure 1: An example of the effect of dipping (blue trace) or not dipping (green trace) the capillary inlet in a clean water vial after sample injection (courtesy of Beckman Coulter). When the capillary was not dipped in water after sample injection, the sample was stuck at the outside of the capillary. This migrated into the capillary after the run was started and the voltage applied, causing the extra sample peaks just after the proper sample peaks. The sample is reduced IgG and the application is the Beckman Coulter CE-SDS kit. This is a CGE protein sizing application comparable with SDS-PAGE. The protein sample is treated with SDS for denaturisation and β-mercaptoethanol for reduction. The original IgG antibody molecule is thus reduced to its heavy chain (HC) and light chain (LC). Also, some non-glycosylated HC (ng-HC) is visible. Because of the denaturisation, all proteins are covered with equal amounts of SDS per amount of protein, shielding the natural charge of the protein. That means that all proteins have the same charge/size ratio. The capillary is filled with SDS-gel buffer which is a linear polymer that through entanglement creates a molecular size sieve and the separation is size-based, just as in SDS-PAGE. Because of the gel, sample injection is electrokinetic (although hydrodynamic injection is also feasible (5)). Because of the unified charge/size ratio, the electrokinetic injection is discriminative, but not between the different proteins. The function of the internal standard (IS), a 10 kDa protein, is for migration time correction. Quantification is by internal normalization (sum corrected peak areas equals 100 %).
Carry-over is reduced by burning off the polyimide coating from the end, which, of course, should be straight (2).
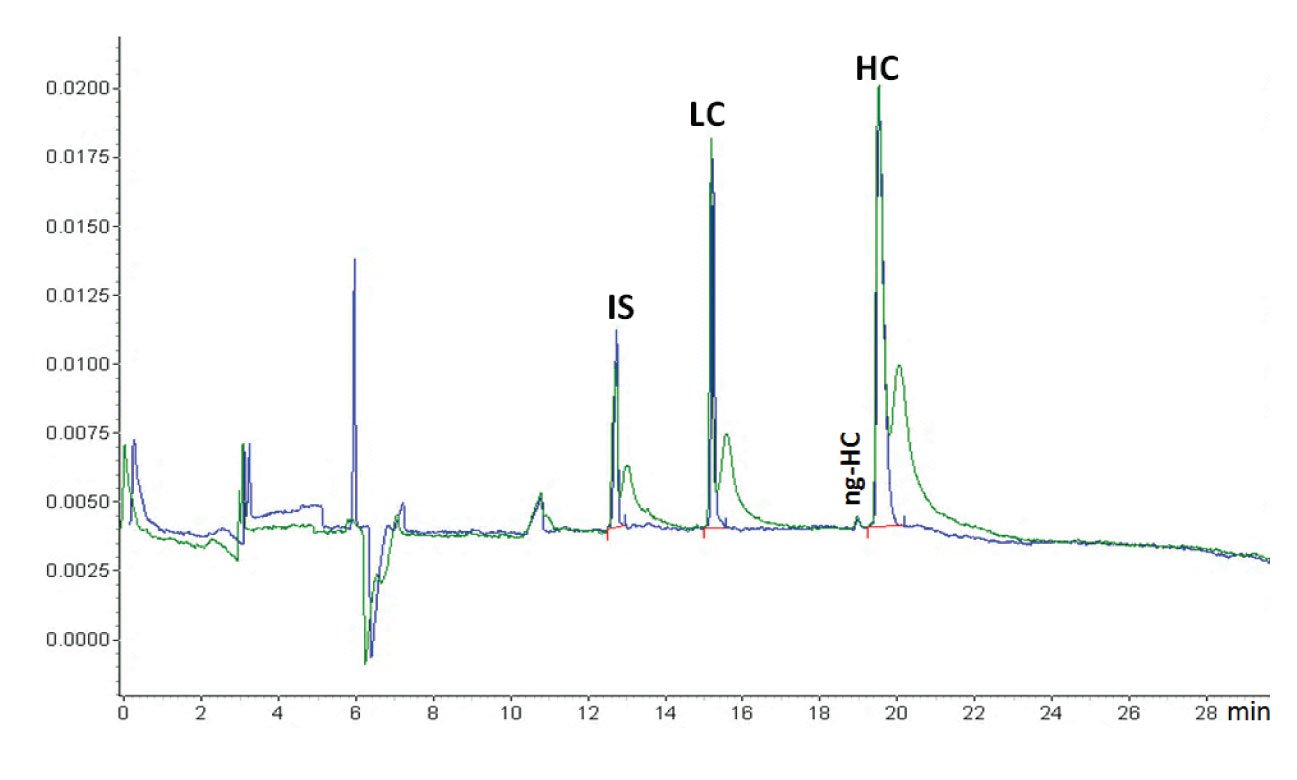
Figure 2: The top trace shows the injection of a standard containing two drug substances (DS 1 and DS 2) and some impurities (Imp a, Imp b and Imp c). The bottom electropherogram shows the injection of an injection solution product containing DS 2 and the impurities spiked in similar amounts as in the standard. The high DS concentration in the product causes migration time shifts and peak broadening (electromigration dispersion) of the later migrating impurities Imp b and Imp c. Imp a is not affected in this system. The broadened, dispersed peaks of Imp b and Imp c are harder to detect and integrate and have lower signal-to noise levels. Lower signal-to-noise levels mean reduced sensitivity. This example demonstrates that method development should preferably be performed with real samples and not just standard solutions.
Samples and Standards
Standards and samples should match in composition. If the standards are simple and clean, low conductive solutions and the samples contain for example lots of highly conductive salts, the resulting electropherograms and peaks can dramatically differ. Even the concentration of the analytes can influence the peak shapes, resolution and migration times of other analytes by electro-migration dispersion phenomena. An example is shown in Figure 2.
For electrokinetic injection, the sample composition affects the ionic strength of the sample. Variable ionic strengths result in variable field strengths during the injection. This means that amount of analyte injected varies from sample vial to sample vial if the matrix is not the same for all samples.
Internal Standard
Even with all these precautions, sample introduction in CE is not as precise as in, e.g., LC. To reduce the effect of injection volume variability further, it is common to use an internal standard (IS). This is especially important when external calibration is used for quantification. For internal calibration (sum of the corrected peak areas equals 100 %) an internal standard is not required. If the function of the internal standard is only to correct for the injection variability of hydrodynamic injections, the IS can be anything that can be detected and does not interfere with the separation at hand.
In electrokinetic injection, an internal standard with similar mobility behaviour as the analytes of interest can be used to reduce injection variability caused by sample composition variability.
Also, correction for migration time variability can be a reason for using an internal standard. For example for identification by relative migration times or relative mobilities or for molecular weight or pI determinations of proteins.
Sample Stacking
Ideally, the injection plug length should be less than 1–2 % of the capillary length. That is if the sample matrix is similar to the BGE. In practice, it is common to make use of stacking mechanisms or online concentration techniques. Stacking reduces the injection bandwidth and increases the sample concentration and with that the sensitivity of the method. In its simplest form, stacking means that the sample is dissolved in a matrix that is at least 10 x less conductive than the BGE, for instance, water or diluted BGE. If dissolving or diluting the sample in water or BGE is not practical, similar effects can be achieved by injecting a water plug before or after the sample plug. When the voltage is applied, the low-conductivity sample zone will have a higher field strength than the BGE. Consequently, the analytes migrate faster until they reach the boundary of the BGE zone. In the more conductive BGE, the field strength is lower and the analytes stack, that is, slow down and concentrate, reducing the initial bandwidth (Figure 3).
There are many other ways in which these basic principles can be applied and in the literature, you will find multiple four and five-letter acronyms for detailed procedures. As I am writing this issue, some very good review articles on this topic have been accepted for publication in Electrophoresis (3, 4). Sensitivity and CE have been very much under discussion in the past years, one of the arguments for reduced sensitivity being the small capillary diameter and therefore the reduced detection path length. As so much can be gained by relatively little effort in the injection procedure, it is important to realise and to stress that detection concentration sensitivity cannot be 1:1 translated to sample concentration sensitivity. The stacking techniques have shown considerable gain over the years and concentration enhancements of 10–1000 times have been achieved.
Good Injection Practice
Summarizing, one can gain significantly in precision and sensitivity if appropriate attention is paid to the injection procedure. Most of the discussed tips and tricks are easily programmed in the method or require very little in the way of sample handling. So except for some time during method development, it is almost for free!
References
1. Capillary Conditioning — The Foundation of Precision in CE, CE Solutions issue 4
2. The CE capillary, CE Solutions issue 3
3. Recent advances in enhancing the sensitivity of electrophoresis and electrochromatography in capillaries and microchips (2010 – 2012), MC Breadmore, AI Shallan, HR Rabanes, D Gstoettenmayr, A Syazwani Abdul Keyon, A Gaspar, M Dawod, JP Quirino, (2012) Electrophoresis doi:10.1002/elps.201200396
4. Contemporary sample stacking in analytical electrophoresis, A Šlampová, Z Malá, P Pantucková, P Gebauer, P Bocek, (2012) Electrophoresis doi:10.1002/elps.201200346
5. Capillary gel electrophoresis for precise protein quantitation, Cianciulli, C., Hahne, T. and Wätzig, H (2012) Electrophoresis doi: 10.1002/elps.201200177
Fill out the form below to access the first module for free of the "Introduction to CE" online course.
Analytical Training Solutions, brought to you by Separation Science, is the leading global portal for fundamentals, best practice, troubleshooting and method development training for chromatographic and mass spectrometric techniques. Comprehensive, self-paced online courses and validated learning provides a unique education resource for analytical chemists. Currently, we offer HPLC training, LC-MS training, GC training and GC-MS training.




